Location:Home >> Detail
Med One. 2017; 2: e170026. https://doi.org/10.20900/mo.20170026
1 Superintendent of Drugs, Directorate General of Drug Administration, Bangladesh
2 Department of Pharmacy, International Islamic University Chittagong, Chittagong-4203, Bangladesh
3 Department of Pharmaceutical Technology, Faculty of Pharmacy, University of Dhaka, Dhaka-1000, Bangladesh
4 Department of Biochemistry and Molecular Biology, University of Chittagong, Chittagong-4331, Bangladesh
Running Head: α-amylase Inhibitory Effects of Phrynium Imbricatum.
Corresponding Author: *Dr. Md. Atiar Rahman, Associate Professor, Department of Biochemistry and Molecular Biology, Faculty of Biological Sciences, University of Chittagong, Chittagong-4331, Bangladesh, Tel: 88-031-2606001-14 (Extn.4334); Fax: 88-031-726310; Cell:+88-01711709084.
Background: Diabetes has become one of the major burdens for developing country people mainly due to excessive cost and adverse effects of synthetic drugs. Plant-derived drugs in Bangladesh is currently being paid an emerging attention because of low cost and diverse floral distribution. This research investigated thein vitroα-amylase inhibitory and hypoglycemic activityof organic etracts of Phrynium imbricatumin normal and glucose induced hyperglycemic mice.
Methods: Extracts were undertaken to measure in vitroα-amylase activity using starch-iodine method while the hypoglycemic effect was studied in glucose induced hyperglycemic mice.
Results: All the extracts of P. imbricatum (EEPI) showed considerable α-amylase inhibitory activity and chloroform extract (CHPI) showed the highest activity among them as compared to Acarbose the reference antidiabetic drug.The extracts at 400 mg/kg and 800 mg/kg b.w. significantly (p < 0.05) reduced fasting glucose level in normal mice as compared to standard drug glibenclamide (5 mg/kg) but CHPI at 800 mg/kg showed the highest hypoglycemic effect decreasing 23.78 % of blood glucose level after 2 h of administration in normal mice, while glibenclamide decreased 49.30 %. In oral glucose tolerance test, at 400 mg/kg and 800 mg/kg of extracts significantly reduced blood glucose level (p < 0.05) at 30 min. Area under the curve (AUC) of the extracts were at the range of 12.895-14.258 hr.mmol/L., and 14.573 hr.mmol/L and 9.835 hr.mmol/L for control and glibenclamide respectively.
Conclusion: The results demonstrate that Phrynium imbricatummay be a very remarkable source for the development of new oral antihyperglycemic agent.
Diabetes is a metabolic disorder usually caused due to either complete or partial dysfunction of insulin secretion with or without varying degrees of insulin resistance [1]. Diabetes involves high blood glucose levels regardless of the type of diabetes. Pancreatic α-amylase constitutes a family of endoamylases which catalyze the cleavages of α-D-(1-4) glycosidic bonds in diabetes incidences [2]. Therefore, inhibition of α-amylase is considered to be one of the major therapeutic strategies for the treatment of diabetes through the retardation of digestion of dietary carbohydrates which are absorbed as monosachharides from intestinal lumen and transported into blood circulation [3]. However, the major drawback of these synthetic inhibitors are their non-specificity in targeting glycosidases eventually produce serious side effects [4]. Thus plant derived drugs are important area of investigation with great potential for discovery of new antidiabetic drugs.Several different medicinal plants and plant-derived drugs have been using for treating diabetes in traditional medicine system as well as in ethnomedicinal practices [6]. This is due to the lesser side effects, cost effective and availability of plant based drugs compared to the synthetic hypoglycemic agents [7]. Additionally, different plants have been reported to show α-amylase inhibitory activity and they may be relevant to the treatment of diabetes [5].
Phrynium imbricatum (Family: Marantaceae) is a herbaceousplant distributed in the forests of Chittagong, Chittagong Hill Tracts, Cox's Bazar and Sylhet. A paste prepared from leaves of P.imbricatum (locally called Khedom gach) is applied to wound and fractures (Chakma) [8]. Leaves of P.imbricatum is as also used as anthelmintic, antiarthritic and membrane stabilizing [8, 9]. This research is designed to investigate the α-amylase inhibitory and hypoglycemic effect of organic extracts (ethanol, chloroform andpetroleum ether)of P. imbricatum leaves in normal and glucose induced hyperglycemic mice.
All the chemicals and reagents used in this research were analytical grade. Ethanol, chloroform and petroleum ether were purchased from Merck, Germany. Α-amylase was procured from Sigma-Aldrich (Sigma-Aldrich Co., USA). Starch, iodine was purchased from Fluka (Fluka chemie GmbH, CH-9471 Buchs). Shimadzu Biospec 1601 UV-visible spectrophotometer (Shimadzu, Japan) was used to measure the absorbance. Rapid ViewTM (Blood glucose monitoring system, Model: BIO-M1, BIOUSA Inc, California, USA) with strips were purchased from local suppliers. Acarbose was procured from the local scientific market, Chowkbazar, Chittagong. Glibenclamide was kindly donated by Square Pharmaceutical Ltd., Bangladesh.
2.2 Plant sample collection and identificationLeaves of P. imbricatum(accession No. 1315 CTGUH)were collected from Alu Tila, Khagrachari, Chittagong, Bangladesh in the month of September 2014 at the late phase of its flowering. It was authenticated by Dr. Shaikh Bokhtear Uddin, Professor and taxonomist, Department of Botany, University of Chittagong, Chittagong-4331, Bangladesh.
2.3 Extraction of plant materialLeaves were cleaned, dried for a period of 10 d under shade and powdered with a mechanical grinder, passing through sieve #40 to store in a tight container. Resulting powder (850 g) was successively soaked in ethanol, chloroform and petroleum ether for 7 d each of 2 d approximately with occasional stirring and filtered through a cotton plug followed by Whatman filter paper number 1. The extract was then concentrated by using a rotary evaporator at reduced temperature and pressure [10]. A total of 55 g of ethanol extract (EEPI), 8 g of chloroform extract (CHPI) and14 g of petroleum ether (PEFPI) was measured to preserve at 4°C for future endeavor.
2.4 Experimental animals and experimental set-upSwiss albino mice of both sexes, weighing about 28-35 g, were collected from the animal house of the Department of Pharmacy, Jahangir Nagar University, Bangladesh. The animals were acclimatized to laboratory condition for 7 d prior to experimentation. They were individually housed in polycarbonated cage at standard laboratory temperature 23 ± 2°C and relative humidity 55-60 % ensuring standard pellete diet and drinking water ad libitum maintaining a regular 12 hr day-night cycle. All the experiments were conducted in an isolated and noiseless condition. The study protocol was approved by the P&D Committee, Department of Pharmacy, International Islamic University Chittagong, Bangladesh. All animal experiments were carried out according to the guidelines of Institutional Animals Ethics Committee and study protocols were approved by the Department of Pharmacy, International Islamic University Chittagong Medical Ethics, Biosafety.
2.5 In vitro α-amylase inhibitory activityThis experiemnt was conducted by a modified starch-iodine protocol described by Hossain et al. [11]. Briefly, 1 mL of plant extract or standard of different concentrations (2, 1, 0.5 mg/mL) was taken in pre-labeled test tubes. The reaction mixture contained 20 μl of α-amylase solution (10 mg/mL), phosphate buffer (0.02 M, pH 7.0) with 0.006 M NaCl (0.4 mL) was added to each test tube and incubated for 10 min at 37 °C. After the incubation, 200 μl of 1 % starch solution was added to each test tube and the mixture was re-incubated for 1 h at 37°C. Then 200 μl of 1 % iodine solution was added followed by adding 10 mL distilled water to each test tube. The absorbance of the mixture was taken at 565 nm. Sample, substrate and α-amylase blank were undertaken under the same conditions. Each experiment was done in triplicate. IC50 value was calculated by using regression analysis:
% α-amylase inhibition = [1- (SA - SBB - SMB)/AAB] × 100
SA = Sample absorbance, SMB = Sample blank, SBB = Substrate blank, AAB = α - amylase blank
2.6 Acute toxicity test and dose selectionTwenty Swiss albino female mice were used for acute toxicity test. Mice were divided into four groups of five animals for each group. Different doses (1000 mg/kg, 2000 mg/kg, 3000 mg/kg and 4000 mg/kg) of EEPI, CHPI and PEPI were administered by stomach tube. Organoleptic changes or any other abnormality of the the animals were recorded for next 24 h. Cage side was observed once daily to note the changes of skin and fur, eyes and mucous membrane, respiratory and circulatory rate, autonomic and central nervous system (CNS). The effective therapeutic dose was taken based on median lethal dose (LD50 > 2.0 g/kg) [12].
2.7 Experimental protocol for in vivo hypoglycemic activity 2.7.1 Hypoglycemic effect in normal miceMice were randomLy divided into eight groups (Group I-Group VIII) each containing five animals. Group I was treated as a control, Group II was treated with glibenclamide (5 mg/kg body weight), Group III-VIII were treated with EEPI and CHPI and PEPI at the doses of 400 mg/kg and 800 mg/kg body weight respectively. Before the treatment, animals were fasted overnight with free access to water and fasting blood glucose levels were measured (Rapid ViewTM blood glucose monitoring system, Model: BIO-M1, BIOUSA Inc, California, USA) [13]. Blood glucose levels were reestimated after 2 h of administration of drug and extract solutions. Percent decrease of blood glucose level after 2 h was calculated using the following equation:
%decrease = (GLbefore - GLafter)/GLbefore x 100
GLbefore = Blood Glucose level before drug or extract administration, GLafter = Blood Glucose level after drug or extract administration.
2.7.2 Hypoglycemic effect of glucose induced hyperglycemic mice (OGTT)Oral glucose tolerance test (OGTT) was performed according to the standard methodwith minor modification [14]. Group I was treated as a normal control group, Group II treated with glibenclamide (5 mg/kg body weight), Group III-VIII were treated with EEPI, CHPI and PEPI at the doses of 400 mg/kg and 800 mg/kg body weight respectively. Sugar solution (1 g/kg body weight) was orally administrated to each animal and the blood glucose levels were measured at 0 (just before sugar ingestion), 30, 60, 90, and 120 min after the administration.Areas under the curves (AUC) for OGTT were calculated to evaluate glucose tolerance [15]. Percent decrease of blood glucose level after 120 min was calculated by the following equation,
% decrease = (GL0 min-GL120 min)/GL0 min x 100
G0 min = Blood Glucose level at 0 min, GL120 min = Blood Glucose level at 120 min
2.8 Statistical analysisData were expressed as the mean ± SEM. The results were analyzed with the software Statistical Package for Social Science (SPSS, Version 22.0, IBM corporation, NY) using One way Aanalysis of variance (ANOVA) followed by Dunnett’s and Bonferroni multiple comparisons when compared against control in OGTT. Paired t-test was performed to show significant variation between before and after blood glucose level. Student’s t-test was performed between IC50 values. Regression analysis was performed to calculate IC50 values. p < 0.05 was considered as statistically significant.
In vitroα-amylase inhibitory action of EEPI, CHPI and PEFI were presented in Table 1. Three of the extracts were found to show very notableα-amylase inhibition. CHPI showed the highest inhibition and it’s IC50was found to be 2.504 ± 0.023 mg/mL which is lower than others and statistically significant (p < 0.001) compared to that of standard antihyperglycemic agent (acarbose) of IC50 0.912 ± 0.015 mg/mL. However, α-amylase inhibitory effects were noted as a dose-dependent phenomenon like Acarbose. The strength of α-amylase inhibitory actions of the extracts were graded as CHPI > EEPI > PEPI.
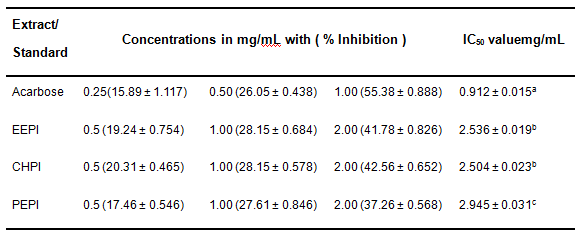 Table 1. IC50 values (mg/mL) for the leaf extracts of P. imbricatum and Acarbose in α-amylase inhibitory assay
Table 1. IC50 values (mg/mL) for the leaf extracts of P. imbricatum and Acarbose in α-amylase inhibitory assay
Values are the mean of triplicate experiments and represented as mean ± SEM (n = 3). Values in the same column with different superscripts are significantly different aP < 0.05, bP < 0.01, cP < 0.001. Student’s t test was performed to analyze this data set.
None of the animals were found to show behavioral, neurological or physical changes characterized by symptoms such as reduced motor activity, convulsions, restlessness, coma, diarrhea and lacrimation at the limit dose of 4000 mg/kg of EEPI, CHPI and PEPI during the observation period. Additionally, no mortality was observed at the test doses. Thus, the median lethal dose (LD50) of the plant extract was found to be greater than 4000 mg/kg. Therefore, the doses 400 mg/kg body weight and 800 mg/kg body weight have been selected for in vivo study (one thenth of LD50) [16].
3.3 Hypoglycemic effect in normal miceHypoglycemic effect of the extracts is presented in Table 2. Both the doses of EEPI, CHPI and PEPI showed a reduction of blood glucose statistically significant (p < 0.05-0.001) compared to glibenclamide. These results suggest the similar significance level for hypoglycemic activity of 800 mg/kg extracts and glibenclamide. The dose of 800 mg/kg of CHPI decreased blood glucose level 23.78 % which was higher than other treatments, except standard glibenclamide.
Values are presented in mean ± SEM (n=6). EEPI=Ethanol extract of P. imbricatum, CHPI=Chloroform extract and PEPI=Pet ether extract. A) Values in same row with different superscripts are significantly different (p < 0.05). Paired t-test was performed to analyze before and after relationship. B) Values with different superscripts in same column are significantly different from control after the administration of standard and different doses of the extracts. One-way ANOVA followed by Dunnett’s multiple comparison was performed to analyze this comparison.
Table 3 describes the oral glucose tolerce data for experimental animals. Induction of hyperglycemia resulted an increased blood glucose level at 30 min of all the treatments and continued till 60 min. Promising reduction of glucose load was recorded for 800 mg/kg of EEPI, CHPI and PEPI at 120 min and their glucose tolerance levels were significant comaperd to (p < 0.05)standard glibenclamide (5 mg/kg). These findings suggest that 800 mg/kg dose is more potent than 400 mg/kg dose. All the data and results of AUC of OGTT are presented in Fig. 1.
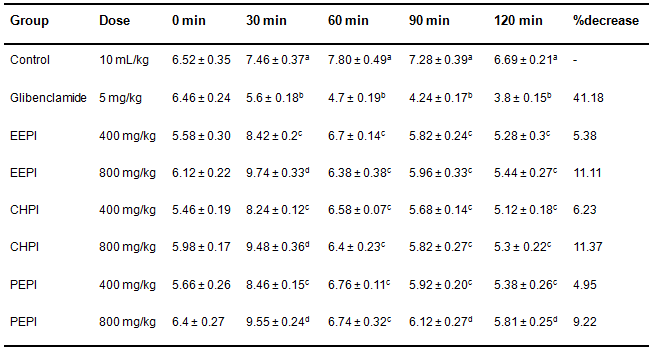 Table 3. Effect of P. imbricatumleaf extracts on glucose induced hyperglycemia (mmol/L) in normal mice.
Table 3. Effect of P. imbricatumleaf extracts on glucose induced hyperglycemia (mmol/L) in normal mice.
Values are presented in mean±SEM (n = 6). EEPI=Ethanol extract of P. imbricatum, CHPI = Chloroform extract and PEPI=Pet ether extract. A) Values with different superscripts in same column are significantly different from control at each specific hour after the administration of standard and different doses of the extracts (p > 0.05). One-way ANOVA followed by Dunnett’s multiple comparison was performed to analyze this comparison. B) Values with different superscripts are significantly different from each other in the same column among standard and different doses of the extracts. Bonferroni multiple comparison was performed.
This research was undertaken to evaluate the hypoglycemic effect organic leaves extract of P. imbricatum, in normal, glucose-loaded hyperglycemic rats. No lethality or no toxicity was found with the selected doses until the end of intervention period. The results of the study have shown that the organic extract of leaves at dose 800 mg/kg has a marked hypoglycemic activity by improvement of the glucose tolerance test in normoglycemic rats.There are multiple options of diabetes while plant based treatment is one of the most popular options now-a-days. This study started with the aim of inhibiting α-amylase, carbohydrate-digesting enzyme, which delays carbohydrate digestion eventually blunting the post-prandial increase of plasma glucose [17-21]. The mechanism involves the inhibition of the conversion of disaccharide to monosaccharide to decrease the rate of entry of glucose into the systemic circulation [22-24].
The oral glucose tolerance test is usually assigned for screening the changes of postprandial glcemia as well as to measure body's ability to use glucose. The extracts P. imbricatum leaves showed marked ability to manage elevated glucose level in normal mice compared to the standard drug glibenclamide. The best glucose tolerance ability of CHPI could be linked with its highest α-amylase inhibitory activity which ensures the higher possibility of best hypoglycemic effect.
Comparison of area under the curve of the treatment groups showed a positive and incremental AUC (r2 = 0.0965) for some of the groups indicating the strong correlation of treatment with glycemic rise. The mechanism by which this leaf extracts exerted this outcome may be due to its action on carbohydrate binding regions of α-amylase enzymes that catalyze the hydrolysis of the internal α-1,4 glucosidic linkages in starch and other related polysaccharides have also been targeted for the suppression of postprandial hyperglycemia [25,26]. Therefore, this and previous study support the claim that natural inhibitors from dietary plants have α-amylase inhibitory activity and could be used as successful therapy for the management of postprandial hyperglycemia with least side effects.
We can conclude that P. imbricatum may have both α-amylase inhibition activity and hypoglycemic effect. It is a rational deduction that this plant may recover the metabolism of glucose and increase insulin secretion by stimulating beta cells. It is possible to propose that the bioactive compounds present in the leaf extracts may be responsible for versatile effects. However, further co-ordinate and well-structured studies would be required to isolate the bioactive compounds and determine their underlying molecular mechanism of action on diabetes-induced mice model. These findings suggest that the plant may be a potential source for the development of new oral hypoglycemic agent.
DM: Diabetes Mellitus;
EEPI: Ethanol extract of P. imbricatum;
CHPI: Chloroform extract;
PEPI: Pet ether extract;
OGTT: Oral glucose tolerance test;
h: Hour;
min: Minutes;
sec: Second;
kg: Kilogram;
g: Gram;
Μg: Microgram;
L: liter;
mL: Millilitre;
µL: Micro liter;
μg/mL: Microgram per Milliliter;
mg/kg: Milligram per kilogram;
mmol: millimole;
IC50: half maximal inhibitory concentration;
LD50: Median lethal dose;
et al.: et alliori (and others);
SEM: Standard error for mean.
This work was carried out in collaboration with authors. Authors MSHK and MMH collected the plant leaves and prepared the extracts. MAR and MSHK carried out the study design, data interpretation, manuscript preparation and statistical analysis. MSHK, MMH, SKS, MSR and MRH participated in experiments, data collection, and literature search. MAR supervised the study design and data interpretation. Author JAC and HA helped in data interpretation and manuscript preparation. All authors read and approved the final version of the manuscript.
The authors are grateful to the authority of International Islamic University Chittagong, Bangladesh, for providing the facilities to conduct this research work. The authors are also thankful to all members of GUSTO (A research group), specially to Nishan Chakrabarty (Department of Pharmacy, IIUC) and Muhammad Abdulla Al Noman (Department of Pharmacy, USTC) for their kind help in the experiment.
The authors declare that they have no conflicts of interest.
1.
2.
3.
4.
5.
6.
7.
8.
9.
10.
11.
12.
13.
14.
15.
16.
17.
18.
19.
20.
21.
22.
23.
24.
25.
26.
Habib M R, Mohammad Shah Hafez Kabir, Rahman M M, Hossain M M, Ali H, Chowdhury J A, Rahman M A. In Vitro α-amylase Inhibitory Potential and in Vivo Hypoglycemic Effect of Organic Extracts of Phrynium Imbricatum Roxb. Leaves. Med One. 2017 Nov 29; 2: e170026. https://doi.org/10.20900/mo.20170026

Copyright © 2020 Hapres Co., Ltd. Privacy Policy | Terms and Conditions