Location: Home >> Detail
TOTAL VIEWS
Regen Med Front. 2019;1:e190005. https://doi.org/10.20900/rmf20190005
1 Department of Biomedical Sciences and Medicine, University of Algarve, 8005-139 Faro, Portugal
2 Centre for Biomedical Research (CBMR), University of Algarve, Campus of Gambelas, 8005-139 Faro, Portugal
3 ABC—Algarve Biomedical Centre, 8005-139 Faro, Portugal
* Correspondence: José Bragança, Tel.: +351-289-800-900 (ext. 302317).
This article belongs to the Virtual Special Issue "Stem Cell Biology and Cell Reprogramming"
The CITED family of transcriptional modulators plays multiple roles in the development of vertebrates. These proteins are characterized by the conservation of a carboxy-terminal domain which defines this family and has a high affinity for the transcriptional co-activators CBP/p300. In humans, mutations in some CITED genes or deregulation of their expression are associated with developmental anomalies. In particular, CITED2 dysfunction has been associated with congenital heart disease. Although most studies have reported the critical function of CITED proteins during development, there is increasing evidence supporting an important role for these proteins in physiological functions of adult organisms and pathologies such as cardiomyopathies and cancer. In addition, recent studies have pointed out the involvement of CITED proteins in embryonic and adult stem cells self-renewal, and cell fate decisions. Here, we present an overview of the CITED transcriptional modulators, their protein interactors and gene networks, with an emphasis on their importance in pluripotent stem cells and cardiogenesis.
CITED, Cbp/P300 Interacting Transactivator With ED Rich Carboxy-Terminal Domain; CHD, congenital heart defects; ESC, embryonic stem cells; TGF, transforming growth factor; CH1, cysteine-histidine-rich domain-1; HIF, Hypoxia-Induced Factor; Nrg, Neuregulin; GRN, gene regulatory network; FGF, fibroblast growth factors; TGF, tumour growth factor; LIF, leukemia inhibitory factor; EpiSC, Epiblastic Stem Cells; BMP, Bone Morphogenetic Protein; iPSC, induced Pluripotent Stem cells; MEF, mouse embryonic fibroblasts
The CBP/p300 interacting transactivator with ED tail rich (CITED) family members are transcriptional modulators present in vertebrates, which bind with high affinity to the transcriptional co-activators and acetyltransferases CBP/p300 [1–8]. During mouse embryogenesis, CITED proteins play pleiotropic roles, and are required for many developmental and morphological processes. Indeed, Cited1 is important for placental development, early nephrogenesis and growth of the embryos [9,10]. Cited2, which is the most studied gene of the family to date, is critical for embryo survival and growth during gestation and for the correct cardiac, adrenal, neural tube, liver, lung, eye, skeletal, thymus, hematopoietic and placenta development [1,2,11–24]. In humans, dysregulation of CITED2 expression and mutations were associated with congenital heart defects (CHD)[1,11,25–27]. In addition to cardiac pathologies, neonatal diabetes mellitus and insulin resistance in adults [28,29], and idiopathic premature ovarian failure affecting woman fertility were also observed [19]. Unlike Cited1 and Cited2 which are critical for development, Cited4 is dispensable for embryonic development since Cited4-knockout mice are viable and fertile [30]. Interestingly, Cited4 expression in adult rodent hearts is associated with healthy cardiac hypertrophy during exercise training [31]. Finally, the non-mammal Cited3 member, which has only been described in chicken (birds) and fish, is also involved in the correct development of these organisms [32–35]. Compelling evidence has indicated that premature senescence of Cited2-null mouse embryonic fibroblasts [36], embryonic stem cells (ESC) self-renewal and differentiation defects due to Cited2 depletion [37,38], as well as death and multiple organ dysfunctions in Cited2-null embryos [39], may be rescued by supplementation or ectopic expression of human CITED2 protein. Like in mouse, a recent study in zebrafish has showed that Cited2 depletion from 1-cell stage triggers the increase of early embryonic death and developmental defects, including cardiovascular defects [40]. Interestingly, in this non-mammalian model, the defects caused by Cited2 depletion can also be rescued by microinjection of a recombinant human CITED2 protein. Thus, the human CITED2 protein shares interchangeable functions (and protein homology) with mouse and zebrafish endogenous Cited2 proteins, arguing that CITED2 protein function is conserved across vertebrates. A similar conservation of other CITED proteins’ function remains unfounded. Several reports have now pointed out the expression and the role of CITED proteins in stem cells (including ESC), in which they are involved in self-renewal and cell fate decisions [38,41–46]. Not surprisingly, due to the plethora of cellular and embryonic functions exerted by CITED1, CITED2 and CITED4, these genes were also showed to play intricate roles in tumorigenic processes [5,45,47–59]. Therefore, CITED proteins are truly important for many embryonic developmental processes and stem cells biology, and their dysfunction may be involved in the establishment and/or the progression of pathological conditions. Here, we review the role of CITED proteins in mouse and human pluripotent stem cells, cell reprogramming, cardiogenesis and adult heart function.
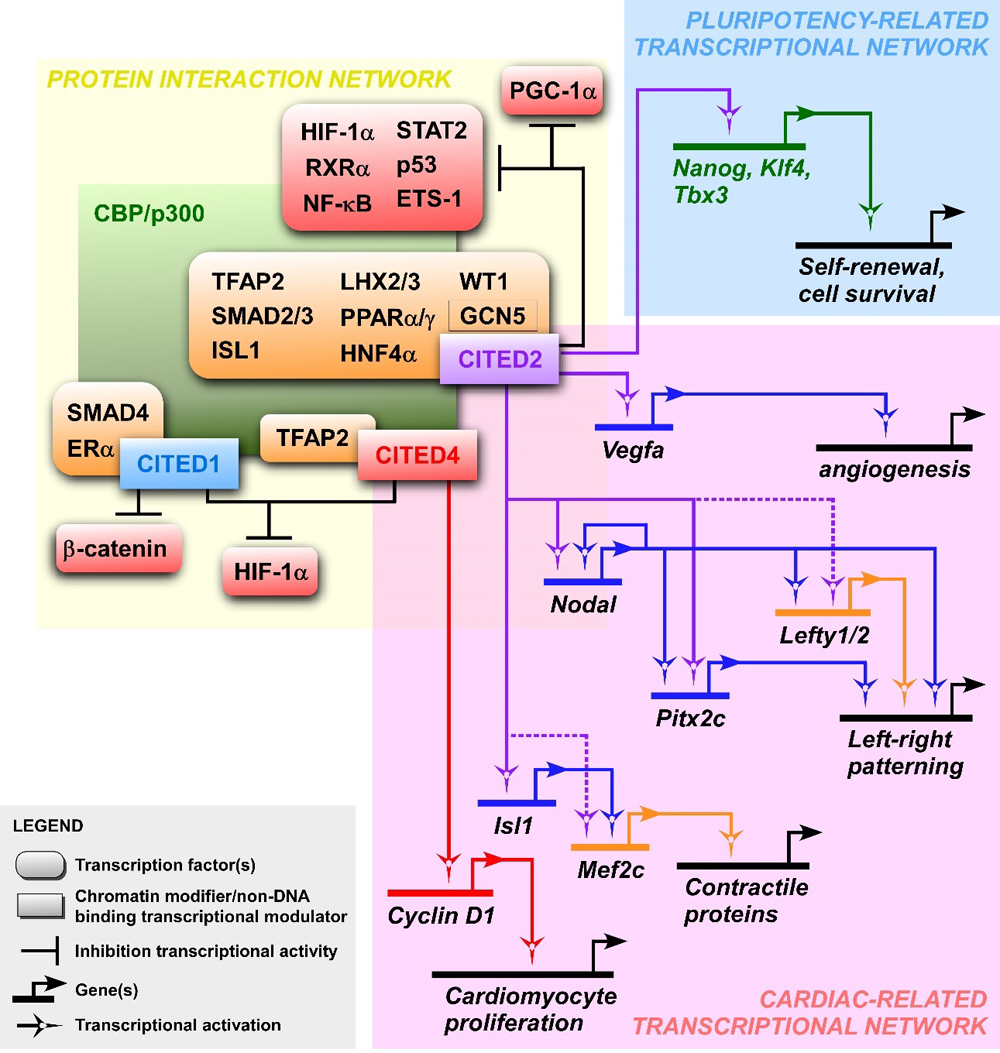 Figure 1. CITED protein interactions and gene regulatory networks involved in the maintenance of pluripotency and cardiogenesis. The yellow panel presents interactions between CBP/p300, CITED proteins and known CITED-interactors. The inhibitory effect of CITED proteins on the transactivation of the indicated transcription factors is represented by inhibitory lines. The absence of inhibitory lines indicates that CITED proteins co-activate the interacting transcription factors indicated. The blue and pink panels show circuit diagrams representing respectively the pluripotency- and cardiac-related transcriptional networks in which Cited2 and Cited4 are involved. The solid lines indicate regulations that have been supported experimentally, while dashed lines represent connections between CITED proteins and the indicated gene regulatory regions suggested by genetic interactions.
Figure 1. CITED protein interactions and gene regulatory networks involved in the maintenance of pluripotency and cardiogenesis. The yellow panel presents interactions between CBP/p300, CITED proteins and known CITED-interactors. The inhibitory effect of CITED proteins on the transactivation of the indicated transcription factors is represented by inhibitory lines. The absence of inhibitory lines indicates that CITED proteins co-activate the interacting transcription factors indicated. The blue and pink panels show circuit diagrams representing respectively the pluripotency- and cardiac-related transcriptional networks in which Cited2 and Cited4 are involved. The solid lines indicate regulations that have been supported experimentally, while dashed lines represent connections between CITED proteins and the indicated gene regulatory regions suggested by genetic interactions.
CITED proteins do not bind directly to DNA and act as co-activators or inhibitors of a variety of transcription factors that require a cooperation with CBP/p300 for their optimal transcriptional activity (Figure 1 and Table 1). The C-terminal domain of CITED is a transactivation domain unique to this protein family, which exhibits a high binding affinity to the cysteine-histidine-rich domain-1 (CH1 domain) of CBP/p300 [7]. Through the interaction with CBP/p300, all CITED proteins may compete out other transcription factors from the CH1 domain, such as the Hypoxia-Induced Factor-1α (HIF-1α), and consequently inhibit HIF/hypoxia-mediated responses [4,6,7,60,61]. In addition to HIF-1α, CITED2 also negatively regulates the activity of other factors interacting with the CH1 domain, such as RXRα, NF-κB, STAT2, p53 and ETS-1 [7,62–67]. CITED1 has been showed to repress β-catenin upon activation of Wnt signalling, and to be a co-activator for Smad4 and estrogen-dependent nuclear receptors [6,68,69]. Both CITED2 and CITED4 are also co-activators of the TFAP2 transcription factors family, while LHX2/3, SMAD2/3, PPARα/γ, HNF-4α, WT1 and ISL1 have only been showed to be co-activated by CITED2 [1–3,8,11,37,70–75]. In addition, CITED2 interacts with the acetyltransferase GCN5 and under fasting conditions, CITED2 promotes PGC-1α activation in response to glucagon by inhibiting the acetylation of PGC-1α by GCN5 [73].
At the transcriptional level, Cited2 expression is highly sensitive to a large number of external and internal cellular stimuli, such as hypoxia/ischemia, interleukins, adrenocorticotropic hormones, growth factors, flow shear stress, estrogens, insulin, high glucose concentrations, lipopolysaccharides, strain-induced mechano-transduction through primary cilia, indoxyl sulphate, disturbances or stress in endoplasmic reticulum and DNA damage [3,7,64,72,78–87]. In addition, FoxP1, as well as other transcription factors required for the maintenance of pluripotency, stimulate the expression of Cited2 in mouse ESC [88]. Cited2 expression in the mesoderm is positively regulated by Wnt3/WNT3A [89]. FoxO1 and FoxO3 may also upregulate Cited2 transcripts levels in cardiomyocytes during myocardial infarction [90]. Unlike Cited2, little is known about the stimuli governing Cited1 and Cited4 expression. The regulation of Cited1 expression by hypoxia has been evidenced in fish but remains to be established in mammals [91,92]. Cited1 expression is also modulated by Wnt3a in ESC and by the parathyroid hormone in osteoblastic cells [93,94]. During mouse embryonic development, Cited1 and Hand1 have overlapping expression patterns in trophoblastic tissues and heart ventricles [9,95,96]. Cited1 expression may also be strongly activated in mouse embryonic hearts by Neuregulin (Nrg) 1 through the ERK1/2-MAP kinase pathway, and by the cardiogenic factors Tbx20 and Hand1, but the exact contribution of Cited1 to heart development remains unclear [96–98]. In mouse and human adipocyte progenitors, Cited4 expression is induced by the antidiabetic drug rosiglitazone [99]. Interestingly, Cited4 expression is induced in adult rat hearts by exercise training, suggesting that Cited4 plays a role in adult heart physiology [31,100].
The CITED transcripts and proteins, as well as their functions, are also regulated at the post-transcriptional and post-translational level. Indeed, TGF-β cell stimulation accelerates the post-transcriptional turnover of Cited2 transcripts [101]. At the protein level, CITED1 interaction with p300 and Smad4, and its transcriptional co-activation capacity are decreased through CITED1 phosphorylation during the cell cycle [61]. In vitro, MAP kinases phosphorylate CITED2 to potentiate its capacity to co-activate TFAP2 [39]. The relevance of this post-translational modification at the cellular and developmental level remains unclear. Finally, the stability of CITED2 protein is also tightly regulated in a proteasome-dependent degradation manner [102,103].
CITED Proteins in Self-Renewal of Embryonic Stem Cells and Maintenance of Pluripotent StateESC are derived from cells forming the inner cell mass of the blastocyst, which originate the future organism. ESC have been an invaluable tool to uncover molecular mechanisms of pluripotency, and have been used also as cellular models to understand developmental processes through the study of their differentiation in vitro. In addition, pluripotent human ESC are considered as a versatile source of cells for diverse therapeutic applications.
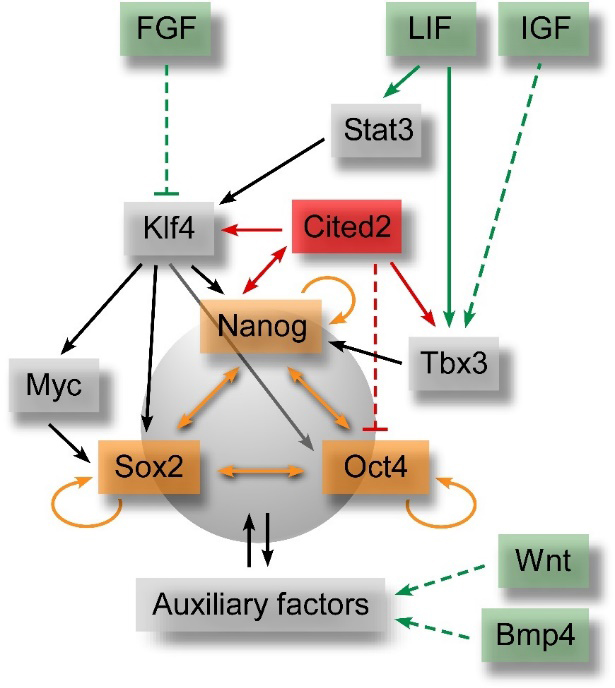 Figure 2. Simplified pluripotency gene regulatory network. Green labels indicate signalling pathways involved in maintenance of pluripotency and self-renewal of pluripotent stem cells. These signalling pathways directly (solid green arrows) or indirectly (dashed green arrows) activate the expression of transcriptional auxiliary factors (grey labels), which interplay and regulate the expression of the core transcription factors (orange labels). The interactions between Cited2 and the pluripotency gene regulatory network are also presented in red. The inhibitory effect of FGF on the expression of auxiliary factors is also indicated.
Figure 2. Simplified pluripotency gene regulatory network. Green labels indicate signalling pathways involved in maintenance of pluripotency and self-renewal of pluripotent stem cells. These signalling pathways directly (solid green arrows) or indirectly (dashed green arrows) activate the expression of transcriptional auxiliary factors (grey labels), which interplay and regulate the expression of the core transcription factors (orange labels). The interactions between Cited2 and the pluripotency gene regulatory network are also presented in red. The inhibitory effect of FGF on the expression of auxiliary factors is also indicated.
Multiple signalling pathways are involved in the self-renewal and maintenance of ESC by activation of Oct4, Sox2 and Nanog expression (Figure 2), which are the core pluripotency master regulators [104,105]. These transcription factors concurrently activate the expression of genes important to maintain the pluripotency state, promote cell proliferation or repress the expression of genes involved in ESC differentiation [105]. The expression of the core pluripotency factors in ESC is stringently regulated, and either an increase or a decrease of their optimal expression levels prompts ESC to differentiate. In mouse ESC, leukemia inhibitory factor (LIF) has been showed to play an essential role in the maintenance of the pluripotent state [106]. LIF binds to cell surface receptors and concurrently activates the JAK/Stat3 pathway to stimulate the expression of Klf4, an activator of Sox2, and the PI3K/AKT pathway to promote Tbx3 expression which consequently activates Nanog expression [107]. In turn, Sox2, Nanog and other auxiliary factors control the expression of Oct4 (Figure 2), and together these factors constitute the pluripotency gene regulatory network (GRN). Wnt/β-catenin and BMP-activated pathways also contribute to sustaining pluripotency of mouse ESC [108–110], while activators of the MAPK/ERK pathways, such as fibroblast growth factors (FGF) stimulate differentiation of mouse ESC [111]. On the other hand, FGF and TGF-β/ACTIVIN/NODAL-SMAD2/3 signalling pathways are critical to maintaining pluripotency of human ESC [112,113]. The transcriptional co-activators CBP/p300 also play significant roles both in the maintenance of pluripotency and the differentiation of ESC, and they are recruited to the regulatory regions of ESC genome in association with Oct4, Sox2 and Nanog [114].
In a Nanog-like fashion, CITED2 overexpression in mouse ESC is sufficient to sustain self-renewal and proliferation in the absence of LIF [38,115]. Conversely, Cited2 acute depletion not only impairs proliferation, but also triggers cell death by apoptosis and spontaneous differentiation of ESC [38]. Unlike Cited2, both Cited1 and Cited4 are poorly expressed in undifferentiated ESC and might not play a major role in the maintenance of the pluripotency [43,46]. Moreover, Cited1 overexpression in mouse ESC triggers their transition to a trophoblast-like state by activation of a trophoblast transcriptional program [43]. In undifferentiated mouse ESC, Cited2 directly controls the expression of Nanog, Tbx3 and Klf4 transcripts and the promoter activity of these genes (Figures 1 and 2, and Table 1), and in turn, Nanog activates Cited2 promoter activity [38]. Surprisingly, a minute population of Cited2-null ESC resisting to cell death and spontaneous differentiation, and presenting characteristics of undifferentiation has been reported [38,76]. However, Cited2-null cells fail to differentiate into cardiac, hematopoietic and neural cell lineages due to a delay in silencing Oct4 expression [76]. An inverse correlation between Oct4 and Cited2 expression levels in mouse ESC has also been reported [116]. These observations suggest that Cited2 is a negative regulator of Oct4 expression in undifferentiated ESC. Additionally, in mouse and human ESC, transcriptional regulators of pluripotency such as Oct4, Sox2, Nanog, FoxP1 and Zfp206 bind the regulatory elements that control the transcription of Cited2 [88,117,118]. Like the core pluripotency transcription factors expression, it appears that Cited2 expression is tightly regulated in ESC by forward and feedback regulatory loops (Figure 2).
In 2007, mouse Epiblastic Stem Cells (EpiSC) were described for the first time and shown to be the mouse cells equivalent to pluripotent human ESC [119,120]. Human ESC and EpiSC are cells concomitantly expressing the core pluripotency factors and transcripts promoting differentiation, unlike mouse ESC (now termed “naïve” or “ground state” ESC) which do not express high levels of genes involved in differentiation. In a sense, EpiSC and human ESC are one step ahead of “naïve” mouse ESC to engage differentiation and are also termed “primed” ESC. Interestingly, hypoxic treatment of mouse “naïve” ESC, or HIF-1α stabilization in these cells, decrease their proliferation rate and drives them to transition from a “naïve” to a “primed” pluripotent state [121–123]. Importantly, hypoxia or HIF, HIF-2α in particular, are important to sustain high proliferation and pluripotency of human ESC by direct activation of the core pluripotency transcription factors expression, such as Oct4 [124,125]. The transition from “naïve” mouse ESC to “primed” EpiSC is associated with the upregulation of glycolytic enzymes important for the production of metabolic energy [121]. Interestingly, Cited2-null mouse ESC display a HIF-1α enhanced-activity which results in the increase of glycolysis and reduction of glucose oxidation [42]. Moreover, the depletion of one HIF-1α allele in Cited2-null mouse ESC rescues some of the defects caused by loss of Cited2 [42]. Thus, one of Cited2 functions in these cells is to attenuate HIF-1α hyper-activity. Interestingly, during mouse ESC differentiation, Cited2 expression decreases from the onset of differentiation until Day 2 (D2) and increases from this time point onwards [37]. Therefore, the decrease of Cited2 expression observed at the onset of differentiation may contribute to establishing a permissive state for mouse ESC transition into an EpiSC-like state by allowing the increase of HIF-1α activity. This hypothesis is corroborated by the fact that high levels of CITED2 ectopic expression, not only promoted self-renewal of mouse ESC, but also drastically impaired their differentiation [37]. After the transition through an EpiSC-like state, in a normal course of differentiation, the rise of Cited2 expression observed from D2 onwards may serve to temper HIF-1α activity and decrease EpiSC self-renewal capacity, to allow EpiSC to enter a new permissive differentiation state and new cell fate specifications.
In early mouse development, Cited1 protein is expressed from 2-cell to blastocyst stages and localizes primarily in the cytoplasm [43]. In blastocysts, Cited1 is mostly detected in the extraembryonic trophectoderm, and its expression in blastocyst structures is inversely correlated to Oct4 expression and activated by BMP4 [43]. These observations are in agreement with the major role played by Cited1 in placental development [9]. In humans and mice, CITED2 expression is gradually increased from one-cell to blastocyst stages with a peak at the blastocyst stage, which matches the expression of core pluripotency factors such as NANOG and SOX2 [126,127]. Together these observations indicate that Cited2 is the only Cited-family member substantially expressed in undifferentiated ESC, and required for self-renewal in vitro and perhaps in vivo. Since the function of CITED2 is highly conserved between mice and humans, it is likely that this protein also plays a critical role in human ESC. Therefore, it would be of interest to clarify the role of CITED2, and other CITED family members in human ESC.
CITED2 and Induced Pluripotent Stem Cells ReprogrammingIn 2006, Shinya Yamanaka and colleagues demonstrated the possibility to originate pluripotent cells (induced Pluripotent Stem Cells, iPSC) from mouse fibroblasts by forced expression of factors Oct4, Sox2, Klf4 and c-Myc, now also named Yamanaka’s factors [128]. iPSC share properties with ESC and can be obtained from the conversion of virtually any somatic cell. Human iPSC were also generated, and represent a promising source of cells for the treatment of many disorders by cell-based therapies and for the discovery of new or patient-personalized pharmacological treatments [129]. Perhaps reminiscent of its role in ESC pluripotency and survival [38,115], CITED2 expression may be critical to originate iPSC. Indeed, Cited2 depletion in immortalized mouse embryonic fibroblasts (MEF) reduced drastically their ability to initiate the reprogramming process [38]. Conversely, CITED2 co-expression with Oct4, Sox2, Klf4 and c-Myc factors increased the efficiency of iPSC generation and accelerated the emergence of iPSC-colonies expressing Nanog [38,130]. Interestingly, the sole overexpression of CITED2 in MEF exposed to LIF is able to unlock the expression of core pluripotency transcription factors such as Nanog, Rex1 and Sox2 [130]. Furthermore, its co-expression with Yamanaka’s factors facilitated the reprogramming process and reduced the variability of some core pluripotency-related genes expression in iPSC clones, particularly if derived from pre-senescent MEF which are usually refractory to reprogramming [38,130]. The expression of constitutively active SMAD2/3 along with Yamanaka’s factors accelerated and increased the number of emerging iPSC colonies [131]. Since CITED2 is a SMAD2/3 co-activator, it would be of interest to test whether the co-expression of CITED2, SMAD2/3 and Yamanaka’s factors further improves the reprogramming process [132]. In fact, SMAD2/3 were proposed to be general potentiators of direct somatic cells conversion not only for iPSC, but also to transdifferentiate a given source cell into non-pluripotent cell types with distinct functions, such as mouse B cells into macrophages, myoblasts into adipocytes, and human fibroblasts into neurons [131]. Interestingly, the direct conversion of human dermal fibroblasts into cardiac progenitors by forced expression of MESP1 and ETS2 stimulates the expression of CITED2 in intermediate cells, suggesting that CITED2 expression may participate in the conversion of fibroblasts into cardiac progenitors [133]. Like SMAD2/3, CITED2 may also act as a general potentiator of direct somatic cells conversion into cells with distinct functions. Together these observations suggest that Cited2 function is substantially associated with undifferentiated pluripotent cells, and that it is of crucial importance for the establishment and maintenance of the pluripotent state in vitro.
Role of CITED in CardiogenesisRecent in vitro studies using mouse ESC to unravel the role of CITED proteins in self-renewal, pluripotency, and lineage specification have established that these proteins are also particularly important at several stages of cardiac cell lineage commitment. Of particular interest, a deleterious Cited2 function in undifferentiated ESC or at the onset of differentiation drastically impairs (cardiac) mesoderm specification and cardiomyocyte differentiation [37,40,76]. Alterations of Nanog, Oct4 and/or Sox2 expression in ESC above or below optimal levels required to maintain pluripotency have an impact in cell fate decisions during differentiation [105]. Cited2 controls the expression of core pluripotency transcription factors in ESC, such as Nanog, Oct4, Klf4 and Tbx3 [38,76]. It would of interest to determine whether Cited2 is involved in conditioning cell fate commitment in undifferentiated ESC through the control of the core pluripotent, or by priming of ESC to cardiac cell fate by promoting the transcriptional expression of pro-cardiogenic genes, such as Isl1, Gata4, Nkx2.5, Wnt5a and Wnt11 in these cells, as suggested in recent reports [37,40].
In vivo, although an extensive overlap of expression exists between Cited1, Cited2 and Cited4 in cardiac structures during mouse development [21,37,95,134,135], only embryos with Cited2-knockout in epiblast cells drastically fail to develop normally and die in utero with a myriad of cardiac and non-cardiac abnormalities [1,9,11,13–15,20–24,30,136–141]. Since Cited2 is important for the correct development and vascularization of the placenta, and that normal placental function is required for correct heart development, part of the cardiovascular defects observed in Cited2-null embryos may also be a consequence of placental defects [9,22]. A cardiomyocyte-specific Cited2-knockout revealed that Cited2 is also important for the thickening and vascularization of the myocardium during gestation [13]. On the other hand, Cited1-null mice die immediately after birth due to defects in trophoblast-derived extraembryonic tissues, while Cited4-null mice are viable and fertile, but neither Cited1- nor Cited4-null mice were reported for any apparent heart defect [9,30]. Cited1 expression may be stimulated by Nrg1 and the pro-cardiogenic transcription factors Tbx20 and Hand1 [96–98]. Importantly, mouse hearts maintain a regenerative potential up to a week after birth. Interestingly, the denervation of the heart impairs cardiac regeneration of neonatal mouse after injury, but the regeneration of denerved hearts can be rescued by administration of Nrg1 and nerve growth factor [142]. It would be of interest, to determine whether Cited1 plays any part in the heart regenerative potential of mouse neonatal pups.
At the molecular level, Cited2 amongst all Cited genes, seems to have a unique function which is to restrain HIF-1α hyperactivity during mouse embryogenesis in hypoxic cardiac structures of the developing heart, such as the interventricular septum, outflow tract and atrioventricular canal regions [23,143]. Cited2 also cooperates with Tfap2 and CBP/p300 to positively regulate Nodal, Lefty1/2, Pitx2c and Vegfa (vascular endothelial growth factor a) expression in embryos (Figures 1 and 2, and Table 1), for correct heart development and the specification of the left-right identity [1,11,13,21]. Complementary studies performed in ESC to recapitulate cardiogenesis in vitro have suggested that Cited2 plays additional key roles at earlier stages of cardiac development. Indeed, loss of Cited2 expression in mouse ESC drastically impairs the cardiogenic process, if depleted at the onset of differentiation [37,76]. Moreover, Brachyury, Mesp1, Isl1 expression, and to a lesser extent Gata4, Nkx2.5 expression is severely downregulated during the differentiation of Cited2-depleted ESC [37,76]. Remarkably, Cited2 depletion after mesoderm specification, happening around D2 of ESC differentiation, does not affect the cardiogenic process [37]. Furthermore, Cited2 depletion in either Brachyury or Mesp1 expressing cells of mouse embryos does not overtly affect heart development [13], corroborating the idea that Cited2 function may not be as critical after (cardiac) mesoderm specification both in vivo and in vitro. Conversely, Cited2 itself may be important for mesoderm specification, since the depletion of Cited2 in ESC at the onset of differentiation decreases Brachyury and Mesp1 expression which are pan-mesoderm and cardiac mesoderm markers, respectively [37]. Concordantly, high expression levels of Cited2 have been detected in early mesoderm Mesp1-positive cardiac precursors [144]. Interestingly, mesoderm progenitors originated from human ESC and iPSC also express CITED2 [145]. These in vivo and in vitro observations suggest that cardiogenic defects due to Cited2-loss of function are essentially a consequence of early mesoderm specification defects, and that CITED2 may be critical for mesoderm progenitors specification. The molecular mechanisms by which Cited2 contributes to early mesoderm specification, and its function in mesoderm progenitors are still unknown.
In addition to its role in early mesoderm specification, Cited2 may have other functions at later stages of cardiogenesis, since its expression is enriched in cardiac progenitor cells in vivo and in vitro [37,144,146]. Moreover, CITED2 is a co-activator of the transcription factor ISL1, which is crucial to originate cardiac progenitors of the secondary heart field (SHF) [37]. Accordingly, it was demonstrated that the combined overexpression of ISL1 and CITED2 in ESC with a low propensity to differentiate into cardiomyocytes increases their cardiogenic potential [37]. Unlike Cited2 which is expressed in ESC and throughout differentiation although at variable levels [37], Cited4 expression has only been detected in ESC-derived cells after the establishment of Brachyury transient expression [46]. Like Cited2, Cited4 is expressed at high levels in cardiac progenitor cells derived from ESC, its expression is transiently increased from approximately D6.5 to D8.5 during differentiation and the overexpression of either Cited2 or Cited4 in ESC stimulates cardiac differentiation [37,46]. Thus, the roles of Cited2 and Cited4 at late cardiogenic steps may be redundant, since the knockout of Cited2 both in vivo and in vitro only drastically affects cardiogenesis when performed before mesoderm specification, at the time when Cited2 is uniquely expressed [13,37]. It would be of interest to determine the precise function of Cited2 and Cited4 in mesoderm and cardiac progenitors. Altogether, these observations suggest that Cited2 plays a privileged role in cardiac development, a role which is not fully interchangeable with other Cited genes.
CITED, Congenital Heart Disease and Adult Heart FunctionNon-synonymous and synonymous variants in CITED2 sequence or reduced expression of CITED2 have been associated with CHD worldwide [25,26,39,147–150]. Ventricular (VSD) and atrial (ASD) septal defects, transposition of the great arteries (TGA) and the tetralogy of Fallot (TOF) are the most frequent heart anomalies associated with CITED2 variants (Figure 3). Most of CITED2 variants identified in patients with CHD, only marginally affect its capacity to repress the transcriptional activity of HIF-1α and/or to co-activate TFAP2 ex vivo [25,26,39,147–150]. However, CITED2 mutations in patients may result in severe impairment of VEGF and PITX2C gene expression, which are necessary for normal development of the vasculature and left-right patterning, respectively [26]. Alternatively, mutations of CITED2 may result in HIF-1α hyperactivity which may hamper cardiac development as demonstrated in mouse animal and ESC models [23]. In any case, since CITED2 cooperates with other transcription factors important for cardiovascular development, such as ISL1 and SMAD2/3 [151,152], the ability of CITED2 mutant proteins to co-activate these factors should also be investigated. Apart from CITED2, only an aberrant hypomethylation of the CITED1 upstream regulatory regions has been detected in monozygotic twins with double outlet right ventricle (DORV), but the role of CITED1 in the pathology remains to be ascertained [153]. To date, no reports have established any association between CITED4 dysfunction and CHD.
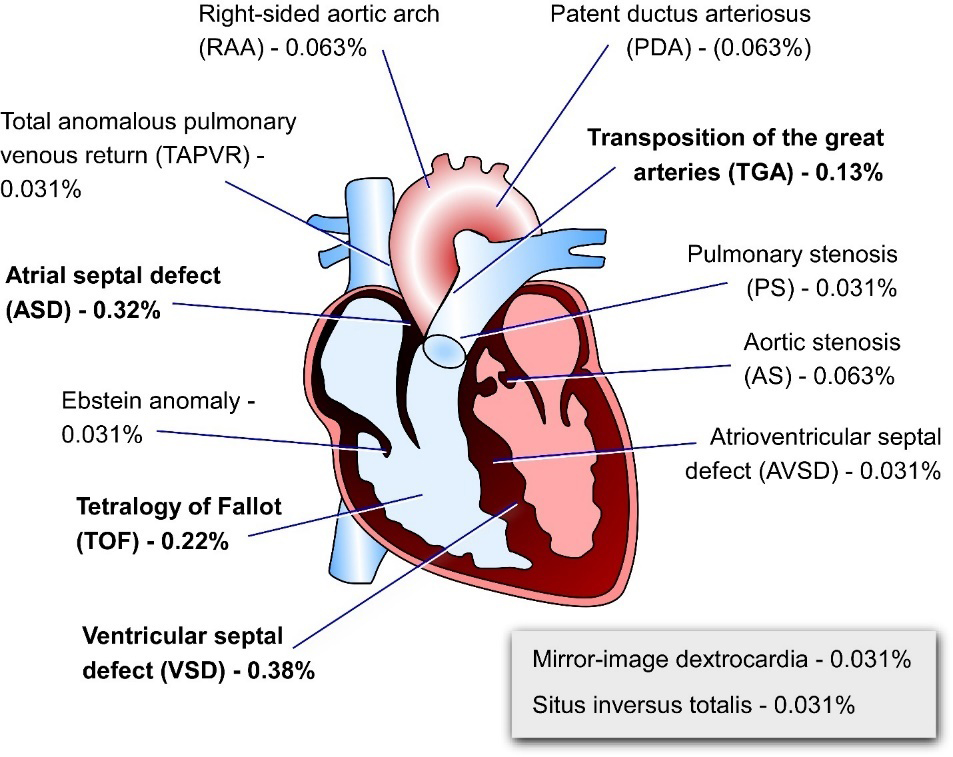 Figure 3. Schematic representation of the adult human heart indicating the heart defects detected in patients of CHD carrying CITED2 mutations. Percentages represent the proportion of each of the heart abnormalities associated with CITED2 mutations in cohorts of patients with CHD. Percentages of defects related to left-right patterning defects are also indicated in the grey insert. Metanalysis of data published elsewhere [25,26,39,147–150].
Figure 3. Schematic representation of the adult human heart indicating the heart defects detected in patients of CHD carrying CITED2 mutations. Percentages represent the proportion of each of the heart abnormalities associated with CITED2 mutations in cohorts of patients with CHD. Percentages of defects related to left-right patterning defects are also indicated in the grey insert. Metanalysis of data published elsewhere [25,26,39,147–150].
In adult hearts, CITED1 has not been detected, while CITED2 and CITED4 are expressed. However, their contribution to heart homeostasis or pathological processes remains largely unclear. During fetal development of mammals, heart muscle grows by hyperplasia due to the increase of cardiomyocyte numbers. In contrast, after birth the cardiac mass increases mostly by hypertrophy, caused by the increase of cardiomyocytes size due to a failure in the cytokinesis after DNA replication and nuclei duplication. Thus, cardiomyocytes in adult mammalian hearts are often binucleated and sometimes multinucleated, although some mononucleated cardiomyocytes persist. The mechanism by which mammalian cardiomyocytes lose their capacity to proliferate soon after birth is still unclear, but an increase in oxygen supply at birth (hyperoxia) has been showed to inflict DNA damage in cardiomyocytes at birth and provoke their cell cycle withdrawal [154]. Hyperoxia at birth may therefore destabilize Hif-1α which is required for proliferation of cardiomyocytes which are in hypoxic conditions during gestation of mammals [155]. To which extend CITED proteins contribute to Hif-1α modulation in cardiomyocytes after birth remains to be investigated. However, in cardiomyocytes isolated from 1-day old neonatal rats, Cited2 expression is associated with the inhibition of proliferation of these cells, and its downregulation by action of miR-410 and miR-495 microRNAs results in proliferation of cardiomyocytes [77]. In these cells, Cited2 has been showed to increase p57 cell cycle inhibitor expression, and decrease Vegfa expression [77]. Thus, Cited2 expression in neonatal cardiomyocytes may promote cell cycle arrest, while in other cell types and during development, its expression is associated with cell proliferation rather than cell cycle arrest. In contrast, Cited4 promotes rat neonatal cardiomyocytes proliferation, but its expression is negatively regulated by the p27 cyclin inhibitor and the transcriptional repressor Hmbox1 [156]. The expression of mir-222 is sufficient to increase CITED4 expression and contribute to cardiomyocytes proliferation [156]. Interestingly, in early neonatal cardiomyocytes Cited2 promotes cell cycle withdrawal while and Cited4 may have and opposing roles and promote proliferation. The interplay between Cited2, Cited4 and Hif-1α, as well as the importance of Cited2 and Cited4 for the establishment of the post-mitotic state of cardiomyocytes remains to be established. Nevertheless, these reports enforce the importance of the regulation of Cited2 and Cited4 expression by microRNAs in cardiomyocytes proliferation. Understanding the contribution of CITED proteins in the shift from a hyperplastic to hypertrophic cardiomyocyte state may paved the way to design novel strategies to regenerate damaged heart structure by stimulation or reactivation of endogenous cardiomyocyte proliferation.
CITED2 transcript levels were showed to be decreased in patients suffering from ischemic cardiomyopathy compared to control individuals [157]. In these patients, a positive correlation between left ventricular function and CITED2 expression has been established. Thus, the downregulation of CITED2 expression in ischemic hearts is likely to exacerbate the chronic maladaptive activation of the HIF pathway and HIF-mediated metabolic switch, which may contribute to cardiac degeneration and progression to heart failure caused by energy deprivation [157–159]. Together, these observations suggest that CITED2 expression preserves the function of adult hearts during adverse ischemic cardiomyopathy.
Recent reports have evidenced the role of Cited4 in healthy exercise-effected cardiac hypertrophy and cardiomyocyte proliferation in rodents through activation of Cyclin D1 expression [31,100]. This healthy hypertrophy is mostly due to a Cited4 autonomous function, since adult transgenic mouse with cardiomyocyte-specific overexpression of Cited4 displayed cardiomyocytes with a larger size, and hearts with increased weight and normal systolic function, resembling the physiologic cardiac hypertrophy induced by exercise [160]. Healthy cardiac hypertrophy through Cited4 expression is controlled negatively by the transcription factor C/EBPβ and positively by miR-222 [31,156]. Cited4 may also function to restrict the elongation size of rat cardiomyocytes to physiological parameters and avoid an excessive and dysfunctional hypertrophy of these cells [100,160]. In the context of ischemia-reperfusion injury in mouse adult hearts, Cited4 overexpression does not protect against the initial insult (infarct size is similar to control animals), but facilitates the functional cardiac recovery and increases animal survival, by reducing adverse ventricular remodelling and fibrosis [160]. Thus, in animal models, Cited4 is important for the maintenance of a healthy adult heart and for recovery of heart function after injury. It would be of interest to determine whether CITED4 also has these functions in human hearts. Overall, CITED2 and CITED4 play distinct, but fundamental roles, in adult cardiac homeostasis, adaptation to exercise-induced demands, and protection against cardiovascular pathological insults. However, the complex and subtle regulation of CITED2 and CITED4, particularly CITED2 with its variety of protein interactors, complicates the prediction of their contribution to heart pathological situations.
The CITED proteins are transcriptional modulators which interplay with multiple DNA-binding transcription factors. CITED transcripts and proteins expression are tightly regulated by diverse signalling pathways and cell stimuli. These factors also share molecular properties, such as the capacity to bind the co-activators CBP/p300 and to inhibit hypoxia/HIF-activated cellular responses. On the other hand, each CITED factor clearly presents particularities, such as the modulation of the activity of specific transcription factors and the regulation of different target genes. Their expressions differ also according to cell-types and spatio-temporal expression in embryos. These latter points were clearly exemplified by studies performed to understand the role of Cited genes in mammalian embryogenesis. Indeed, Cited2-loss of function in mouse leads to embryonic lethality in utero in association with a variety of developmental defects including heart malformations, adrenal agenesis amongst the most persistent anomalies. On the other hand, Cited1 which has an expression pattern that overlaps with those of Cited2 in embryonic heart is dispensable for cardiac development. Nevertheless, Cited1 is required for kidney development and survival of mouse pups after birth, while Cited4-loss of function in embryos does not affect mouse development, viability and fertility in adults.
Developmental studies of mouse embryos also revealed that Cited2 is important for mesoderm specification, and for myocardium thickening and vascularization, septation of heart chambers and outflow tract formation (OFT). In addition, Cited2 dysfunction also affects left-right patterning and heart looping. Interestingly, Cited2 stimulates the expression of Isl1, a transcription factor which is a hallmark of cardiac progenitors of the SHF, a group of cells contributing to the formation of the right ventricle, heart atria and OFT. Furthermore, Cited2 expression is enriched in cardiac progenitors of the SHF in mouse embryos at mid-gestation, and cardiac progenitors derived from ESC. Therefore, it would be of interest to further explore the role of Cited2 in these progenitors. Not surprisingly, several human cardiac congenital abnormalities have been associated with genetic mutations or misexpression of CITED2. However, the exact mechanisms by which mutations in CITED2 affect cardiac development are still poorly understood. The majority of the mutations associated with CHD occur in the CITED2 unique domain caller SRJ (mutation hotspot), but rather surprisingly this domain has been showed to be dispensable for normal mouse embryonic development. Most of CITED2-CHD association studies have determined the effect of CITED2 mutations on the inhibition of HIF-1α transactivation and the co-activation of TFAP2. However, the effects of the mutations were marginal for most of the cases. Recently, CITED2 has been shown to be a co-activator for ISL1, and together ISL1 and CITED2 cooperate to activate the promoter of cardiogenic genes, such as Mef2c, and stimulate the differentiation of ESC to cardiac lineages. It would be of interest to further explore whether a disruption of the ISL1-CITED2 cooperation may be involved in CHD. In adult hearts, the role of Cited2 for cardiac homeostasis and protection against injuries is not well established, while Cited4 has been described as a major factor for healthy heart hypertrophy.
In recent reports, Cited2 has been associated with the stemness of embryonic and adult stem cells. In ESC, Cited2 is an important auxiliary factor stimulating the expression of the core pluripotency transcription factors Nanog, Tbx3 and Klf4, and acting negatively on Oct4 expression. Consequently, Cited2 acute depletion in mouse ESC triggers their death by apoptosis and/or spontaneous differentiation. Cited2 depletion in undifferentiated ESC or at the onset of differentiation, limits ESC full range lineage specification. Therefore, Cited2 expression in ESC is central not only for self-renewal, but also to drive their specification, as showed for mesoderm and cardiac cell fate commitment. A better understanding of the regulatory transcriptional networks contributing to ESC self-renewal, pluripotency, and cell fate decisions involving Cited2 and perhaps other Cited members, would provide invaluable cues to therapeutically address the dysfunction of these proteins in diseases. Reminiscent of its role in ESC, Cited2 is essential for the generation of iPSC from mouse fibroblasts, indicating that Cited2 may have a role in the establishment of pluripotency in vitro. Using CITED2 in cell conversion processes may also improve cellular reprogramming and provide cells with better quality and more suitable for clinical therapies.
Overall, the precise role of CITED proteins in adult organism and in specific tissues or cell-types, including stem cells, remains largely unknown. In the future, a better knowledge of the molecular and specific cellular functions of the Cited genes will contribute to understand their roles in heart development, pathogenic or cardioprotective responses, and to develop novel therapeutic approaches to limit cardiac dysfunctions and/or restore normal cardiac functions.
The authors declare that they have no conflicts of interest.
JB group’s research is supported by national Portuguese funding through FCT—Fundação para a Ciência e a Tecnologia, project UID/BIM/04773/2013 CBMR, FCT and the Comissão de Coordenação e Desenvolvimento Regional do Algarve (CCDR Algarve) for the project ALG-01-0145-FEDER-28044 “VITAL”. LM-S is PhD student of the ProRegeM financed by the FCT.
The present review is dedicated to the memory of Laura A. Bragança (12/05/2003–10/05/2019), who has always been a strong supporter of our work.
1.
2.
3.
4.
5.
6.
7.
8.
9.
10.
11.
12.
13.
14.
15.
16.
17.
18.
19.
20.
21.
22.
23.
24.
25.
26.
27.
28.
29.
30.
31.
32.
33.
34.
35.
36.
37.
38.
39.
40.
41.
42.
43.
44.
45.
46.
47.
48.
49.
50.
51.
52.
53.
54.
55.
56.
57.
58.
59.
60.
61.
62.
63.
64.
65.
66.
67.
68.
69.
70.
71.
72.
73.
74.
75.
76.
77.
78.
79.
80.
81.
82.
83.
84.
85.
86.
87.
88.
89.
90.
91.
92.
93.
94.
95.
96.
97.
98.
99.
100.
101.
102.
103.
104.
105.
106.
107.
108.
109.
110.
111.
112.
113.
114.
115.
116.
117.
118.
119.
120.
121.
122.
123.
124.
125.
126.
127.
128.
129.
130.
131.
132.
133.
134.
135.
136.
137.
138.
139.
140.
141.
142.
143.
144.
145.
146.
147.
148.
149.
150.
151.
152.
153.
154.
155.
156.
157.
158.
159.
160.
Bragança J, Mendes-Silva L, Lopes JA, Calado SM. CITED proteins in the heart of pluripotent cells and in heart’s full potential. Regen Med Front. 2019;1:e190005. https://doi.org/10.20900/rmf20190005

Copyright © 2020 Hapres Co., Ltd. Privacy Policy | Terms and Conditions